The chemical shift of hydrogens is caused by the electron distribution in the molecule the movement of the electrons produce small magnetic fields that affect the net field experienced by each hydrogen nucleus proton.
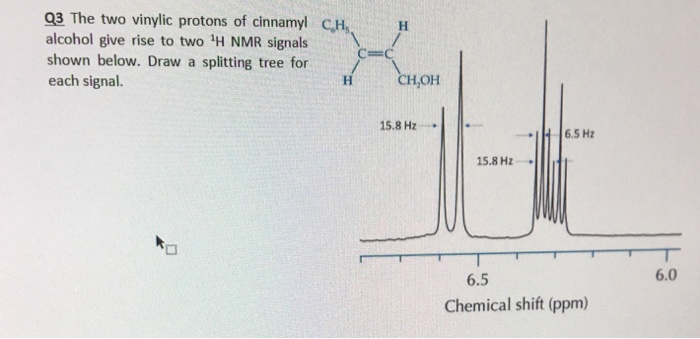
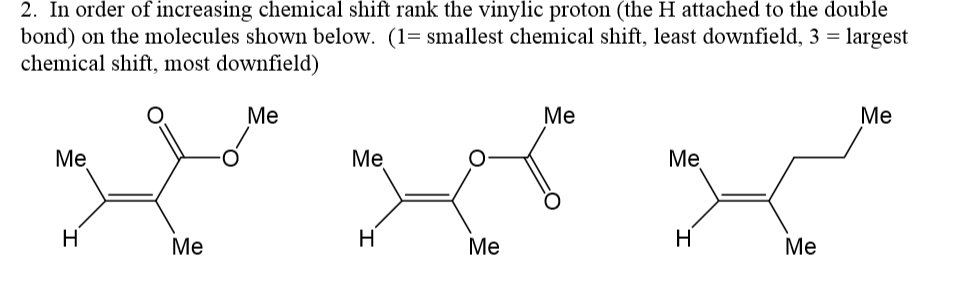
Vinylic hydrogen chemical shift.
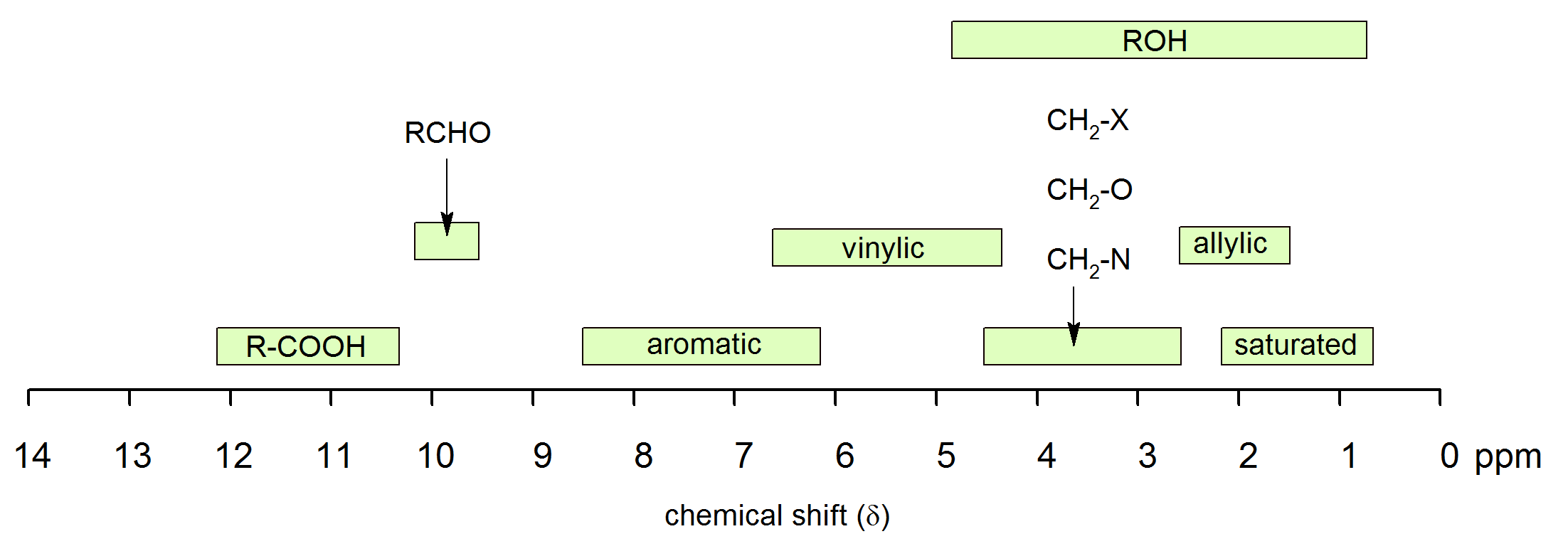
The chemical shifts of aromatic and vinylic protons some protons resonate much further downfield than can be accounted for simply by the deshielding effect of nearby electronegative atoms.
You can also get negative shifts down to about 10.
Vinylic protons those directly bonded to an alkene carbon and aromatic benzylic protons are dramatic examples.
Yes but most shifts are less than those seen for carboxylic acids.
Those bonded to carbon atoms which are in turn bonded to a highly electronegative element.
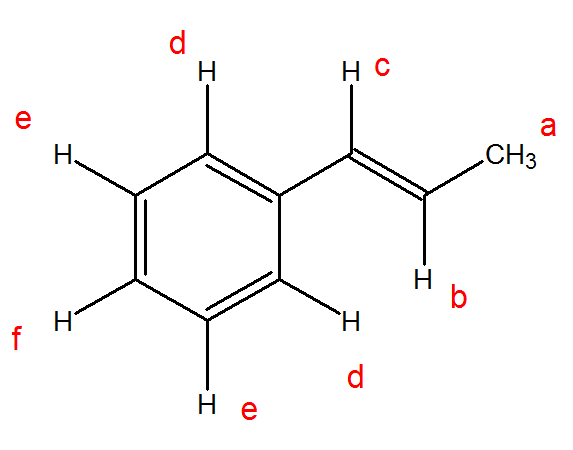
Identify the different equivalent protons in the following molecule and predict their expected chemical shift.
Hydrogens directly bonded to mercury have shifts around 17.
Those bonded to.
The most extreme value is for naked i e completely unshielded protons and those have a shift of about 40.
The following have one h 1 nmr peak.
Table of characteristic proton nmr shifts type of proton type of compound chemical shift range ppm rch 3 1 aliphatic 0 9 r 2 ch 2 2 aliphatic 1 3 r 3 ch 3 aliphatic 1 5 c c h vinylic 4 6 5 9 c c h vinylic conjugated 5 5 7 5 c c h acetylenic 2 3 ar h aromatic 6 8 5 ar c h benzylic 2 2 3 c c ch 3 allylic 1 7 hc f.