Its empirical formula is c 2 h 3 more generally a vinylic cation is any disubstituted trivalent carbon where the carbon bearing the positive charge is part of a double bond and is sp hybridized in the chemical literature substituted vinylic cations are often referred to as vinyl cations and understood to.
Vinylic carbon structure.
The name is also used for any compound containing that group namely r ch ch 2 where r is any other group of atoms.
It contains two sp 2 hybridized carbon atoms and one sp 3 hybridized carbon atom.
For example the following compounds have all been isolated from the volatile oil of chondrococcus hornemanni a red seaweed found in the pacific ocean.
Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
A carbocation in which the carbon atom having the open octet and positive formal charge is part of a carbon carbon double bond.
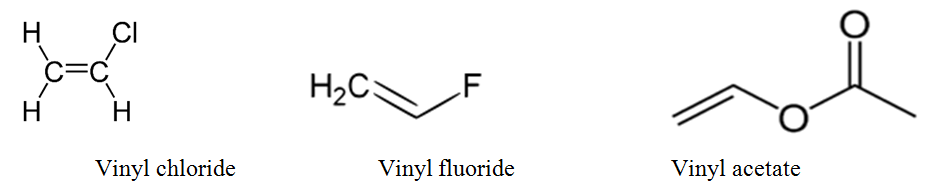
An industrially important example is vinyl chloride precursor to pvc a plastic.
In other words it is a methylene bridge ch 2 attached to a vinyl group ch ch 2.
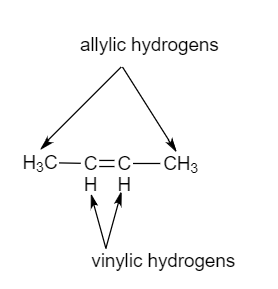
This molecule has four vinyl ic positions each marked with.
The key difference between allylic and vinylic carbon is that allylic carbon is the carbon.
The carbocation carbon has sp hybridization.
Any trivalent disubstituted carbon is generally a vinylic carbocation in which the carbon atom which is bearing the positive charge is found to be double bonded and will always exist as sp hybridized.
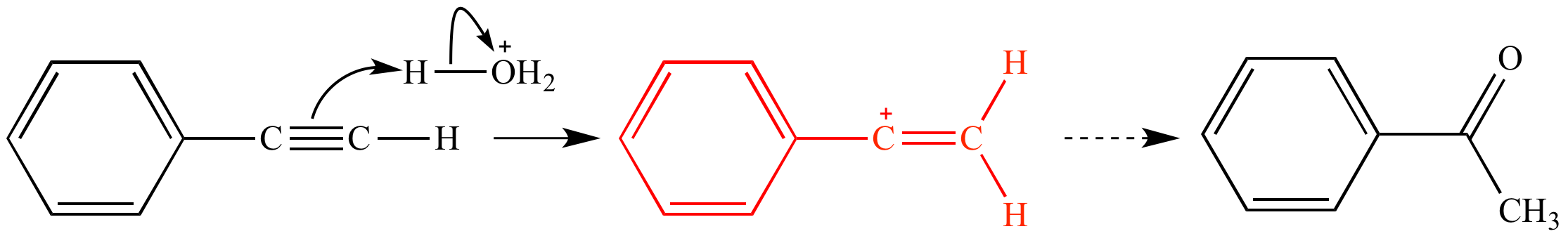
The vinyl cation is a carbocation with the positive charge on an alkene carbon.
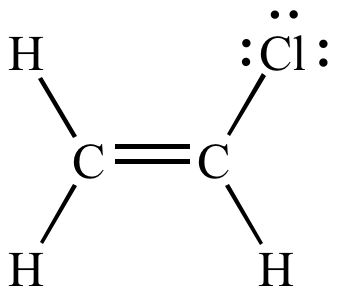
Lewis structure of vinyl chloride a vinyl ic halide.
Vinylic chlorides and bromides constitute a diverse class of marine natural products.
Vinylic halides natural occurrence.
A vinylic carbocation which has an empirical formula of c h is a carbocation that has a positive charge only on the alkene carbon atom.
The general formula for vinyl group is r ch ch 2 in which both carbon atoms are bonded with double bond and r is attached at vinylic position.
General vinylic carbocation structure.
In chemistry vinyl or ethenyl abbreviated as vi is the functional group with the formula c h ch 2 it is the ethylene iupac ethene molecule h 2 c ch 2 with one fewer hydrogen atom.
The double bonded carbon atoms can be classified as vinylic and allylic carbon atoms.
On or bonded to the carbon of an alkene.